Abstract
Ibrutinib in combination with rituximab and high-dose methotrexate in newly diagnosed primary central nervous system lymphoma patients
Yixian Guo, Xiaoxi Lan, Xiaoli Chang, Guoxiang Wang, Dongmei Zou, Li Su, Wanling Sun
Department of Hematology, Xuanwu Hospital, Capital Medical University, Beijing 100053, P.R. China
Corresponding author: Wanling Sun, M.D. & Ph.D.
Email: wanlingsun@xwhosp.org
Background: Primary central nervous system lymphoma (PCNSL) is a rare extranodal lymphoma. Most PCNSL are pathologically classified as diffuse large B-cell lymphoma (DLBCL). In addition to the importance of high-dose systemic methotrexate (HD-MTX), there is no consensus on the optimal composition of induction and consolidation therapy for newly diagnosed PCNSL. BTK inhibitors are the second-line recommended choice in relapsed of refractory cases. In this study, we described our institutional experience with the oral BTK inhibitor, ibrutinib, combined with rituximab and HD-MTX (I-RM regimen) in newly diagnosed PCNSL. This study is registered with Chictr.org.cn, number ChiCTR1900027811.
Methods: The study was a phase 1, investigator-initiated, clinical trial being conducted at Xuanwu Hospital, Capital Medical University . Patients definitely diagnosed as PCNSL-DLBCL by biopsy of brain lesion, systemic imageology, bone marrow and CSF examination, et al, and be suitable for the immunochemotherapy,were enrolled the clinical trial. The next-generatioin sequencing of the lesion tissue were used to detect hot spot mutations evolved in B cell lymphoma. The ibrutinib-based I-RM regimen consists of rituximab 375mg / m 2 on day 0, MTX 3.5g/m 2 on day1, and ibrutinib 560mg once daily starting from plasma MTX concentration <0.1umol/L to day 28, every 28 days is a cycle of treatment. After 4 cycles of I-RM induction therapy, patients who achieved CR/CRu/PR received autologous peripheral blood hematopoietic stem cell transplantation (HSCT) or the extra 2 cylces of I-RM regimen as consolidation therapy. Ibrutinib or lenalidomide monotherapy was prescribed as maintenance therapy, according to the willingness of patients until the disease progresses. The primary end point was the safety and efficacy of this regimen in newly diagnosed PCNSL.
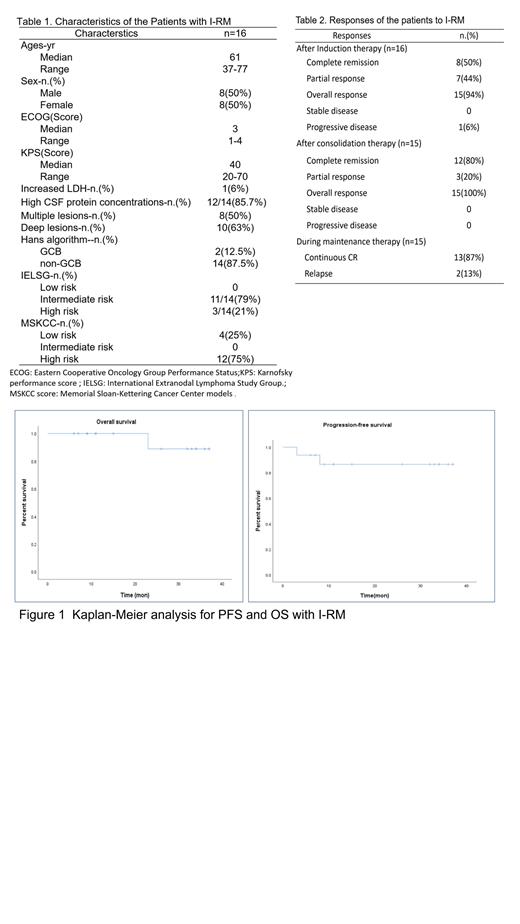
Results:There were totally16 patients enrolled in this clinical trial, between April 2018 and December 2020 (Table 1). All the patients were pathologically DLBCL, including 14 non-GCB subtypes and 2 GCB subtypes. The commonest hot spot mutations were PIM1 (68.75%), MYD88 (43.75%), and CD79B (37.50%). The median follow-up time was 24.5 (6-37) months up to June 30, 2021. After 4-cycles of I-RM induction therapy, CR were achieved in 8 patients (50%), PR in 7 patients (44%), and progressive disease in one patients(6%) who quit the clinical trial and transferred to other regimen,and the ORR of induction therapy was 94%. Among 15 responsers, 5 (33%) received HSCT and 10 (67%) received extra 2 cylces of I-RM regimen as consolidation therapy. After the consolidation therapy, 93% patients (14/15) received maintenance therapy, including 6 received lenalidomide and 8 received ibrutinib. Relapse happened in 2 patients during the maintenance therapy, including 1 death due to the progressed disease (Table 2). The median PFS and OS were not achieved, and the estimated 3-year OS and PFS were 88.9% and 86.5%, respectively (Figure 1). No grade 4 adverse event was observed. The grade 3 hematologic toxicities included lymphopenia in 3 cases and neutropenia in 1 case .The grade 3 non-hematologic toxicities including hypertension in 1 case and increased serum creatinine in 1 case.
Conclusions: Our data preliminarily indicates that I-MR is active and safe as a first-line regimen in newly diagnosed PCNSL and might effectively improve the survival of these patients. More patients and longer follow-up are needed to confirm our conclusion.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal